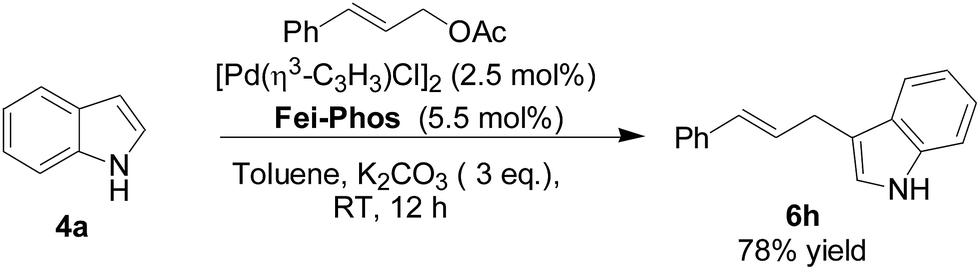
Fei-Phos ligand-controlled asymmetric palladium-catalyzed allylic substitutions with structurally diverse nucleophiles: scope and limitations - RSC Advances (RSC Publishing) DOI:10.1039/C6RA09657C

Palladium allylic complexes with enantiopure bis(diamidophosphite) ligands bearing a cyclohexane-1,2-diamine skeleton as catalysts in the allylic substitution reaction - ScienceDirect

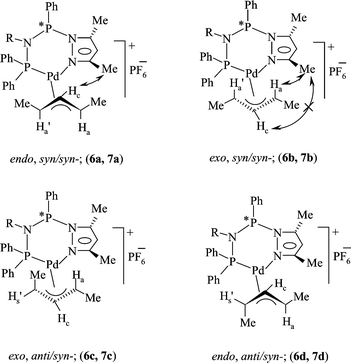
A mechanistic study on multifunctional Fei-Phos ligand-controlled asymmetric palladium-catalyzed allylic substitutions - RSC Advances (RSC Publishing) DOI:10.1039/C6RA15665G

Fei-Phos ligand-controlled asymmetric palladium-catalyzed allylic substitutions with structurally diverse nucleophiles: scope and limitations - RSC Advances (RSC Publishing) DOI:10.1039/C6RA09657C

Preparation of palladium membranes from the reaction of Pd(C3H3)(C5H5) with H2: wet-impregnated deposition - ScienceDirect

Aminoalkyl-phosphine (P,N) ligands with pentane-2,4-diyl backbone in asymmetric allylic substitution reactions | SpringerLink
Palladium( ii ) allyl complexes of chiral diphosphazane ligands: ambident coordination behaviour and stereodynamic studies in solution - Dalton Transactions (RSC Publishing) DOI:10.1039/B207447H

Asymmetric synthesis of chiral cycloalkenone derivatives <i>via</i> palladium catalysis. - Abstract - Europe PMC

Preparation of palladium membranes from the reaction of Pd(C3H3)(C5H5) with H2: wet-impregnated deposition - ScienceDirect

Catalytic deoxygenation of fatty acids and their derivatives to hydrocarbon fuels via decarboxylation/decarbonylation - Santillan‐Jimenez - 2012 - Journal of Chemical Technology & Biotechnology - Wiley Online Library
![Scheme 2 Methods for isolation of [Sr(C 3 H 5 ) 2 ] (1) from K[Sr(C 3 H... | Download Scientific Diagram Scheme 2 Methods for isolation of [Sr(C 3 H 5 ) 2 ] (1) from K[Sr(C 3 H... | Download Scientific Diagram](https://www.researchgate.net/profile/Yann-Sarazin/publication/225300857/figure/fig3/AS:601672183193625@1520461235530/Scheme-2-Methods-for-isolation-of-SrC-3-H-5-2-1-from-KSrC-3-H-5-3-2-All.png)
Scheme 2 Methods for isolation of [Sr(C 3 H 5 ) 2 ] (1) from K[Sr(C 3 H... | Download Scientific Diagram

Deformation density and energy decomposition to describe interactions between (η5-C5H5)M and highly reactive molecules C4H4 and (C3H3)− | Semantic Scholar

Transformation of Carbonates into Sulfones at the Benzylic Position via Palladium-Catalyzed Benzylic Substitution

Preparation of palladium membranes from the reaction of Pd(C3H3)(C5H5) with H2: wet-impregnated deposition - ScienceDirect

Molecular orbital diagram for the model allyl complex 2′, [(PH 3 ) 2... | Download Scientific Diagram

A mechanistic study on multifunctional Fei-Phos ligand-controlled asymmetric palladium-catalyzed allylic substitutions - RSC Advances (RSC Publishing) DOI:10.1039/C6RA15665G









