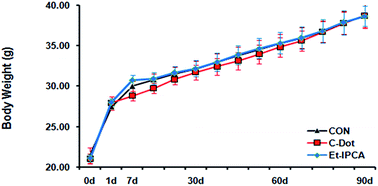
Single and repeated dose toxicity of citric acid-based carbon dots and a derivative in mice - RSC Advances (RSC Publishing)

Repeated-doses and reproductive toxicity studies of the monoterpene 1,8-cineole (eucalyptol) in Wistar rats - ScienceDirect

Acute and repeated doses (28 days) oral toxicity study of Vicenin-1, a flavonoid glycoside isolated from fenugreek seeds in laboratory mice - ScienceDirect
PLOS ONE: Evaluation of 90 day repeated dose oral toxicity and reproductive/developmental toxicity of 3'-hydroxypterostilbene in experimental animals
Description of prototype modes-of-action related to repeated dose toxicity - Publications Office of the EU

Read-across of 90-day rodent repeated-dose toxicity: A case study for selected simple aryl alcohol alkyl carboxylic acid esters - ScienceDirect

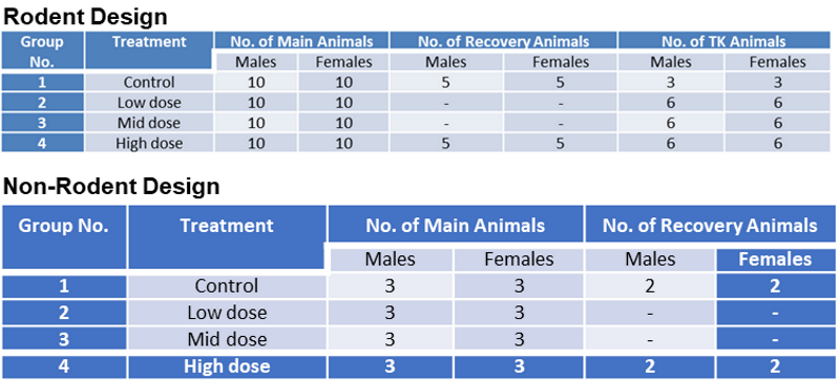
Scheme of repeated dose toxicity study for VGX-6150. C57BL/6 mice were... | Download Scientific Diagram

Foods | Free Full-Text | Single and Repeated Dose 28-Day and 13-Week Toxicity Studies of Oil Prepared from the Internal Organs of the Japanese Giant Scallop (Patinopecten yessoensis) in Mice

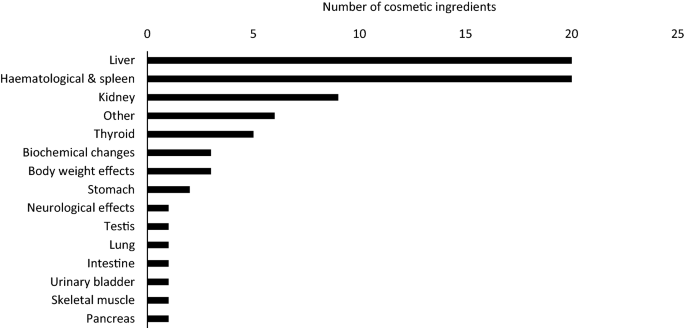
Table 1 from Screening of repeated dose toxicity data present in SCC(NF)P/SCCS safety evaluations of cosmetic ingredients | Semantic Scholar
![PDF] Screening of repeated dose toxicity data in safety evaluation reports of cosmetic ingredients issued by the Scientific Committee on Consumer Safety between 2009 and 2019 | Semantic Scholar PDF] Screening of repeated dose toxicity data in safety evaluation reports of cosmetic ingredients issued by the Scientific Committee on Consumer Safety between 2009 and 2019 | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/8b5f0afa1b6be636ff89730381ba807f7ba2ec86/3-Table1-1.png)
PDF] Screening of repeated dose toxicity data in safety evaluation reports of cosmetic ingredients issued by the Scientific Committee on Consumer Safety between 2009 and 2019 | Semantic Scholar
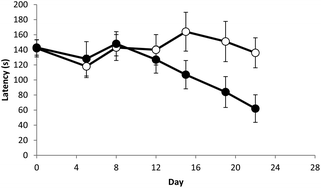
Functional assessments in repeat-dose toxicity studies: the art of the possible - Toxicology Research (RSC Publishing)

Figure 1 | Modes-of-Action Related to Repeated Dose Toxicity: Tissue-Specific Biological Roles of PPARγ Ligand-Dependent Dysregulation in Nonalcoholic Fatty Liver Disease