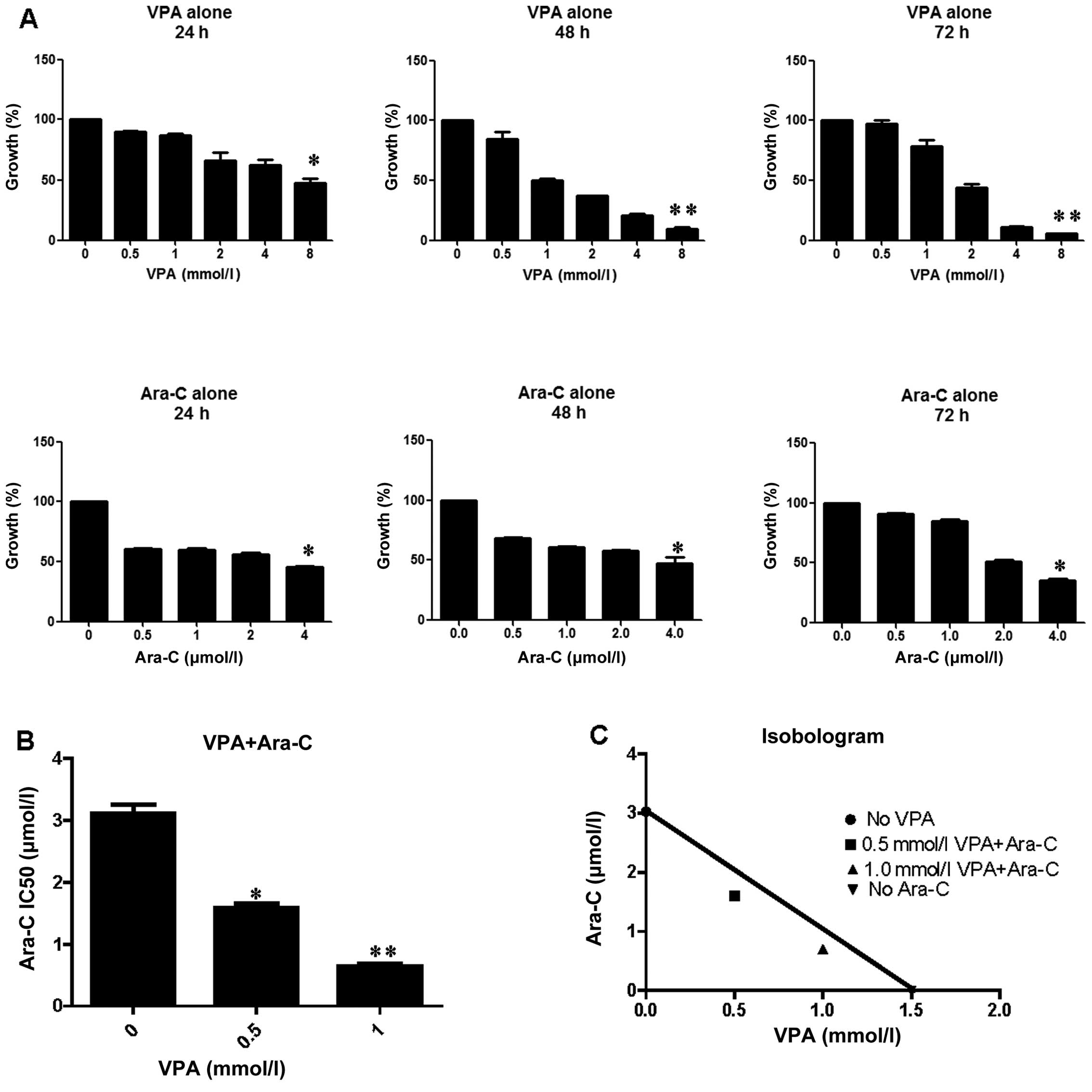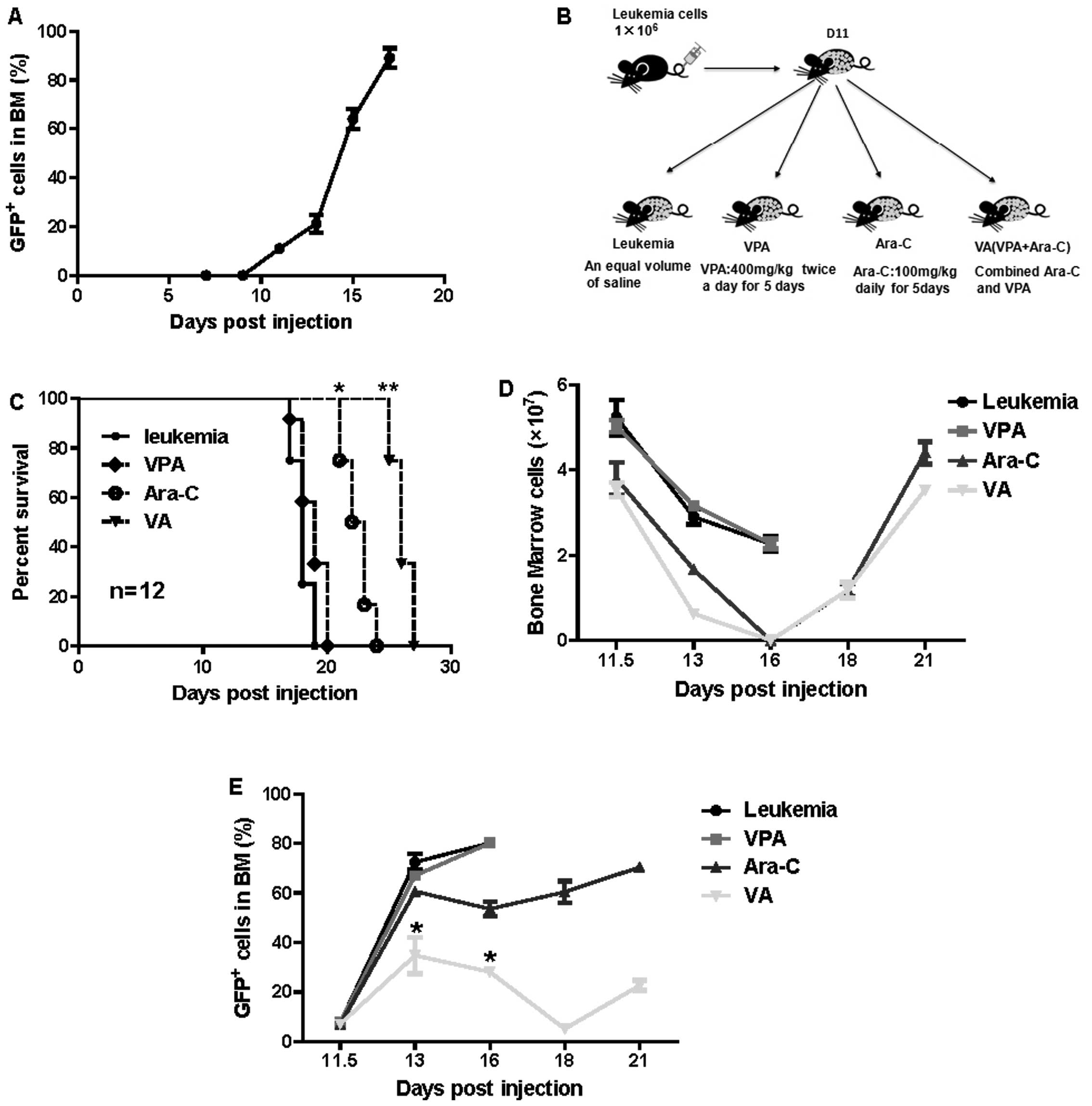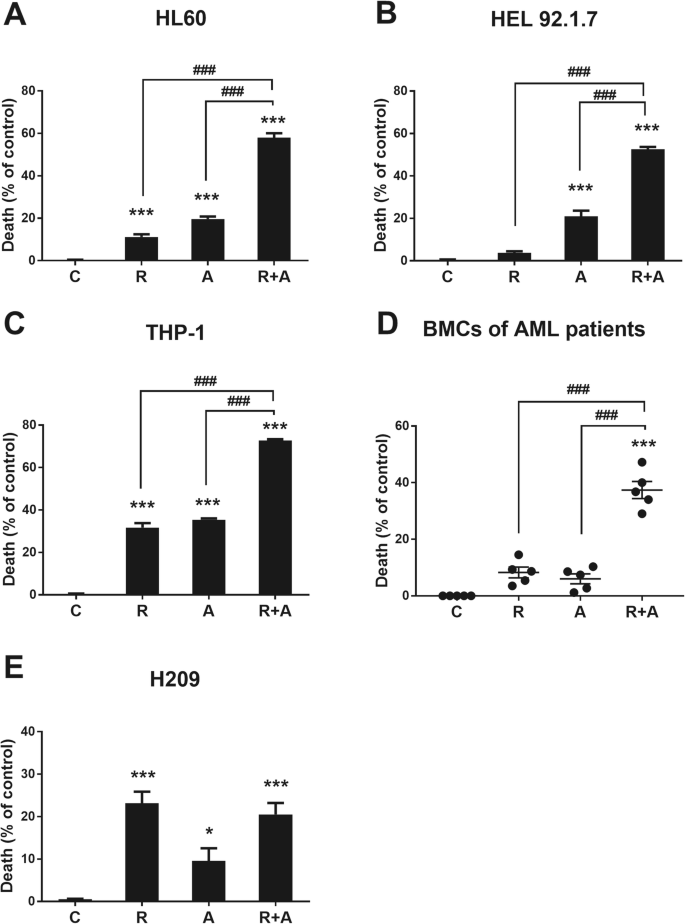
Radotinib enhances cytarabine (Ara-C)-induced acute myeloid leukemia cell death | BMC Cancer | Full Text

The effects of cytarabine combined with ginsenoside compound K synergistically induce DNA damage in acute myeloid leukemia cells - ScienceDirect

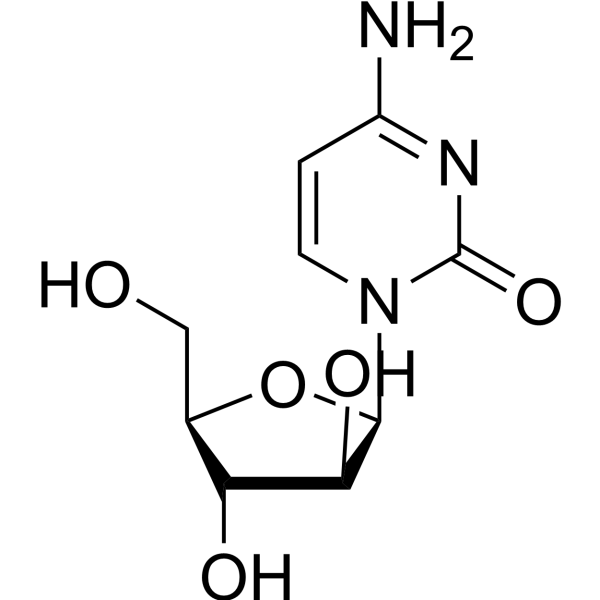
Cytarabine (1-β-D-Arabinofuranosylcytosine, NSC 63878, NSC 287459, U-19920A, CAS Number: 147-94-4) | Cayman Chemical
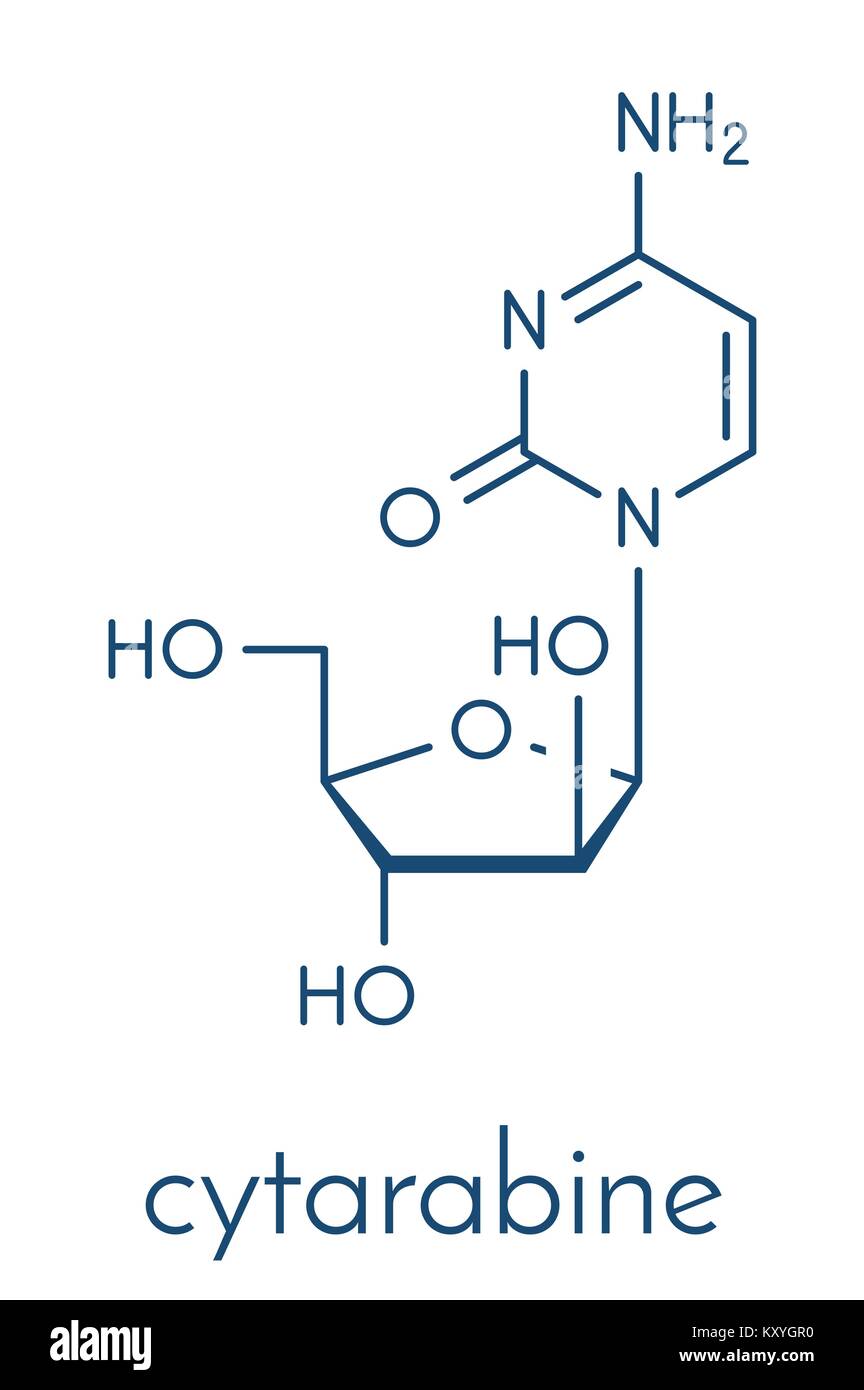
Cytarabine (cytosine arabinoside, Ara-C) chemotherapy drug molecule. Used in treatment of acute myeloid leukemia (AML), acute lymphocytic leukemia (AL Stock Vector Image & Art - Alamy

Phase I and pharmacokinetic study of elacytarabine, a novel 5′-elaidic acid derivative of cytarabine, in adults with refractory hematological malignancies | Leukemia

Neurotoxicity of cytarabine (Ara-C) in dorsal root ganglion neurons originates from impediment of mtDNA synthesis and compromise of mitochondrial function - ScienceDirect

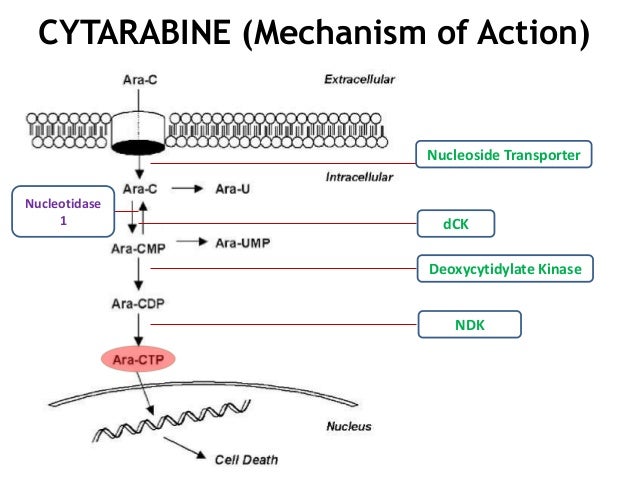
Ribonucleotide reductase inhibitors suppress SAMHD1 ara‐CTPase activity enhancing cytarabine efficacy | EMBO Molecular Medicine
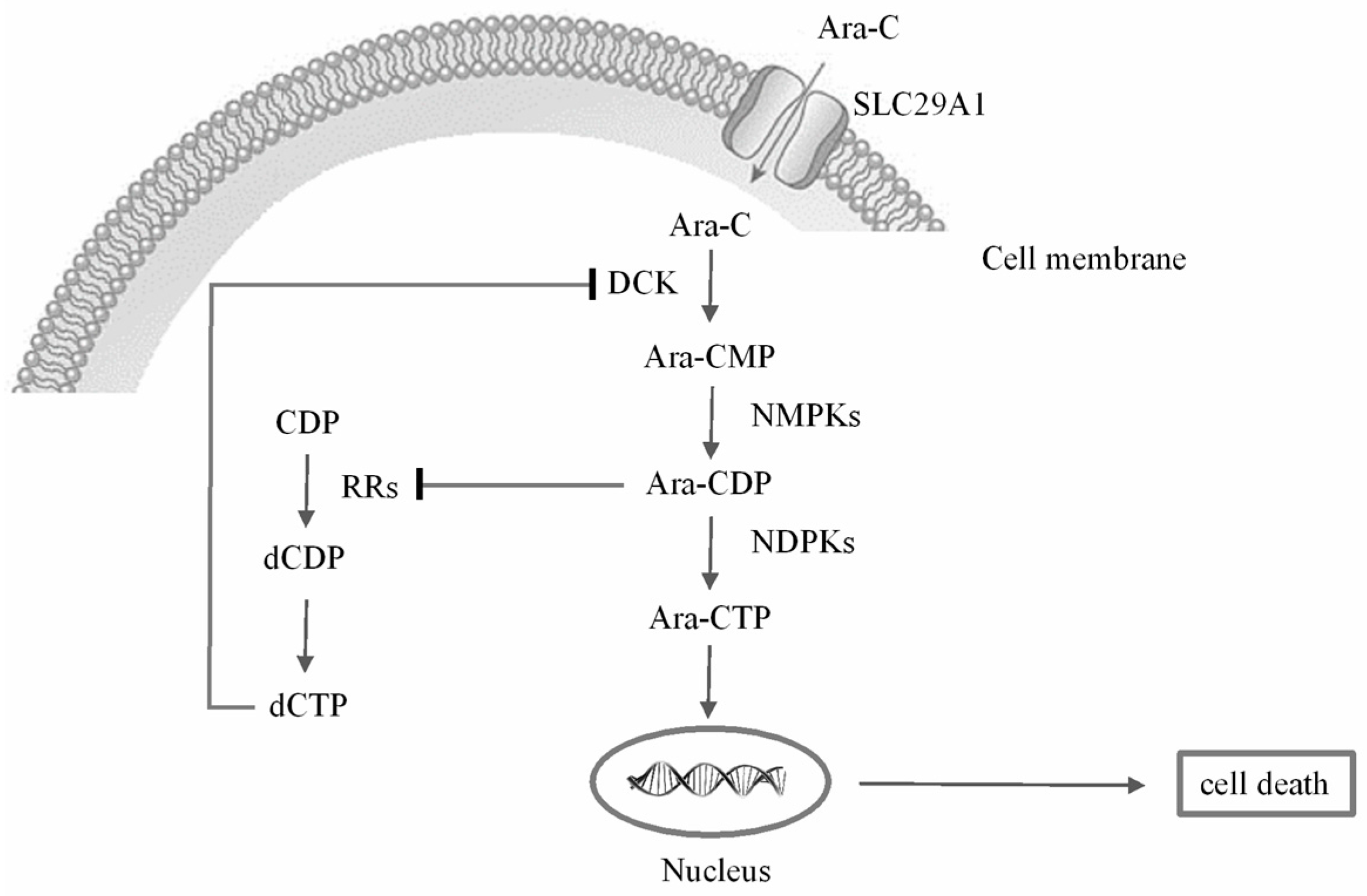
Molecules | Free Full-Text | Exploring the Antitumor Mechanism of High-Dose Cytarabine through the Metabolic Perturbations of Ribonucleotide and Deoxyribonucleotide in Human Promyelocytic Leukemia HL-60 Cells | HTML